[ad_1]
Two varieties of quinapril and hydrochlorothiazide tablets made by Aurobindo Pharma USA are being recalled as a result of they comprise too-high ranges of nitrosamines. These compounds are generally present in water and meals — together with meats, dairy merchandise and greens in decrease ranges — however might improve the danger of most cancers if individuals are uncovered to them above acceptable ranges over lengthy durations of time. That is based on the voluntary recall from Aurobindo, which was posted by the US Meals and Drug Administration.
Quinapril and hydrochlorothiazide are used to deal with hypertension, or hypertension. Two a lot of Aurobindo’s 20 mg/12.5 mg are included within the recall, each with expiration dates via January 2023, and the particular lot numbers could be discovered within the recall announcement on Oct. 25. The tablets are pink-colored and spherical, with “D” printed on one facet and “19” on the opposite facet.
When you’ve got this remedy, it is best to speak along with your physician earlier than you cease taking it, the discover posted by the FDA mentioned. Untreated hypertension can elevate the danger of stroke, coronary heart assault and different critical well being points or medical emergencies, so it is best to focus on the dangers and advantages of discovering one other remedy.
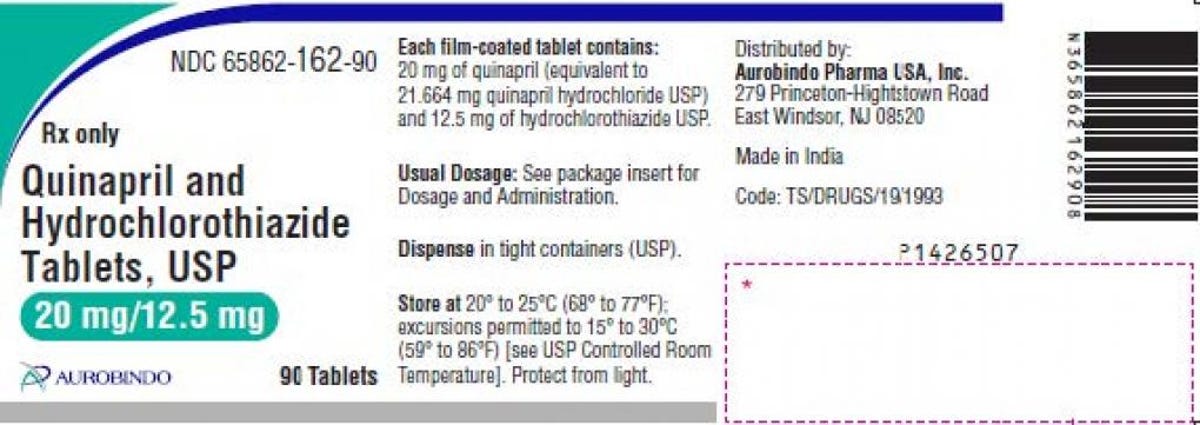
The hypertension remedy label supplied within the recall.
FDA
To this point, Aurobindo mentioned, it hasn’t obtained any experiences of adversarial occasions associated to the recall. Customers with medical questions in regards to the recall can contact the corporate at 866-850-2876 (possibility 2) or e-mail Aurobindo at pvg@aurobindousa.com. For questions on returns, customers can name 888-504-2014.
Different blood stress medicines below completely different manufacturers have been recalled this 12 months over the identical concern about excessively excessive nitrosamine ranges. As The New York Instances reported, the “wave” of remembers is probably going on account of a rise in testing for issues like nitrosamines. Many drug or beauty remembers over potential contamination or impurities aren’t meant to trigger alarm or to say customers will probably be instantly harmed when utilizing a recalled product. As a substitute, the priority is usually over frequent use over an extended time frame.
The data contained on this article is for instructional and informational functions solely and isn’t supposed as well being or medical recommendation. At all times seek the advice of a doctor or different certified well being supplier concerning any questions you might have a few medical situation or well being aims.
[ad_2]
Source link



